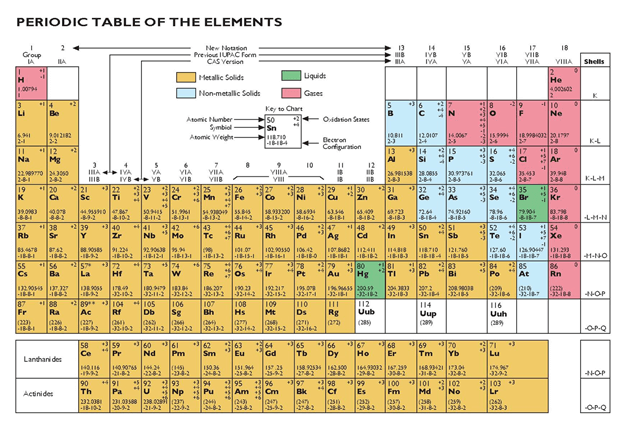
A chemical element can be defined as a substance in matter that cannot be split or converted into another element using any chemical means. A chemical element is also a member of the atom family that has a specific quantity of protons in the atomic nuclei. These elements are responsible for the structure of matter. There are more than a hundred elements known in science, which are categorized in a periodic table. A periodic table is a display of chemical elements. They usually are displayed according to their atomic number, chemical components, and name, to name a few. For instance, a periodic table of elements in alphabetical order is arranged according to the title. In this post, we discuss the importance of periodic tables.
- Classification of elements
The primary role of the periodic table is to identify the elements that make up matter. Scientists and researchers discover new matter in different parts of the world all the time. The periodic table, therefore, comes in handy when the time comes when the matter needs to be identified. Scientists use the chart to break down the atomic structure of the matter and compare it with the other elements categorized on the table. The table also helps researchers to predict the behavior of the matter in its original state and when combined with other elements.
- Identification of elements
A periodic table is an essential tool for identifying chemical elements. The reason for this is that it describes different elements according to their structure. The table also helps to identify the atomic elements, the number of electrons, weight of the elements as well as how the different chemical elements compare. Therefore, if you have an atomic number and need to know the name of the element, you can cross-reference the number on the periodic table.
- Categorizing the elements
Another vital role of the periodic table is to categorize the elements in families and periods. The families and periods consist of items that share similar characteristics and a distinction of those that do not have the same features. Items that share the same atomic weight and structure also fall in the same category. Elements categorized together also behave similarly when they are introduced to certain chemical conditions.
- Predicting the properties of elements
The periodic table is also used to predict the properties and behaviors of individual elements. This benefit is also applicable to elements that have not been discovered yet.
- Balancing chemical equations
Scientists and students use the periodic table to balance chemical equations. This happens thanks to the vital information that is present on the periodic table, as the number of electrons. It is, therefore, a powerful learning tool in science.
- Take Away
Typically, periodic tables are organized to showcase various trends such as the atomic radius, ionization energy, electron negativity as well as the electron affinity. The periodic table is generally a tabular or graphic collection of different information on elements. The rows on a periodic table are known as periods, and the columns are called groups.